
HTRF Human Phospho-IRAK4 Thr345 Detection Kit, 500 assay points
HTRF Human Phospho-IRAK4 Thr345 Detection Kit, 500 assay points


This HTRF kit allows for the cell-based quantitative detection of IRAK4 when phosphorylated at Thr345.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit allows for the cell-based quantitative detection of IRAK4 when phosphorylated at Thr345.


HTRF Human Phospho-IRAK4 Thr345 Detection Kit, 500 assay points


HTRF Human Phospho-IRAK4 Thr345 Detection Kit, 500 assay points


Product information
Overview
IRAK4 is a protein kinase belonging to the interleukin-1 receptor associated pathway (IRAKs) that plays a critical role in initiating innate immune response against pathogens.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Automation Compatible |
Yes
|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
IRAK4
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Inflammation
Oncology
Rare Diseases
|
| Unit Size |
500 assay points
|
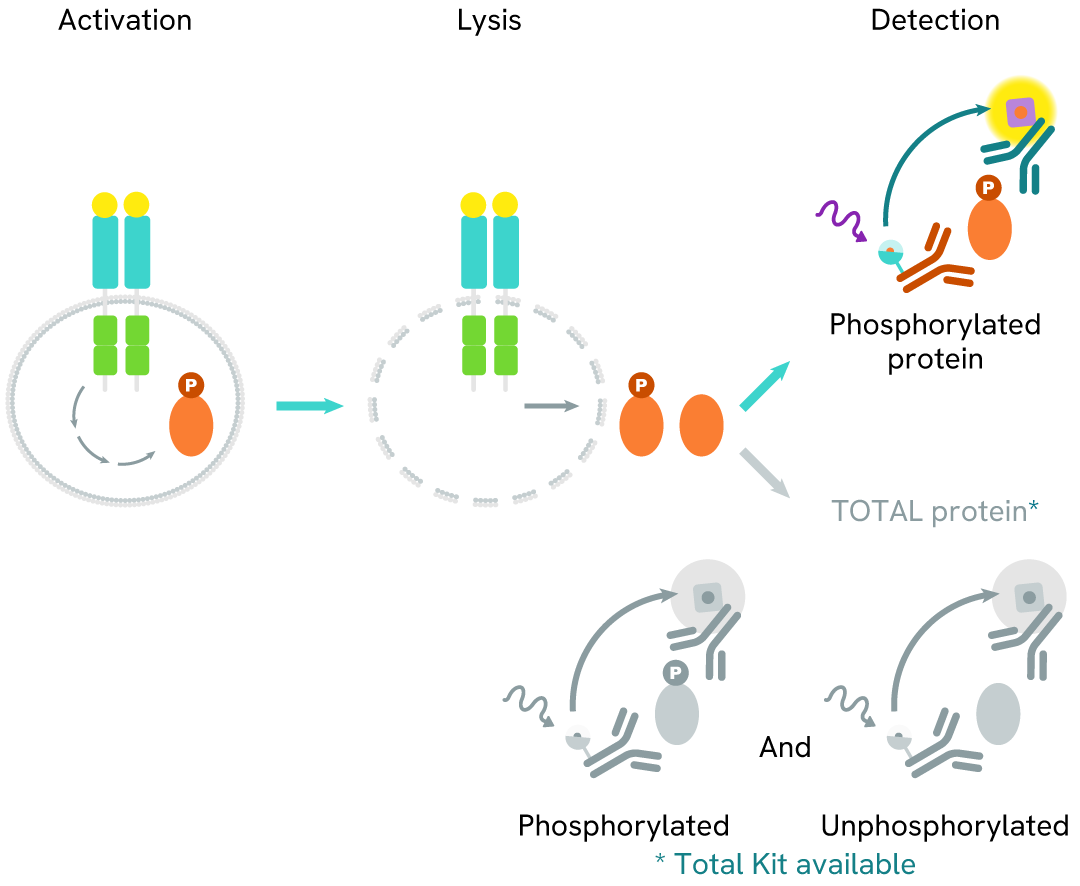
How it works
Phospho-IRAK4 (Thr345) assay principle
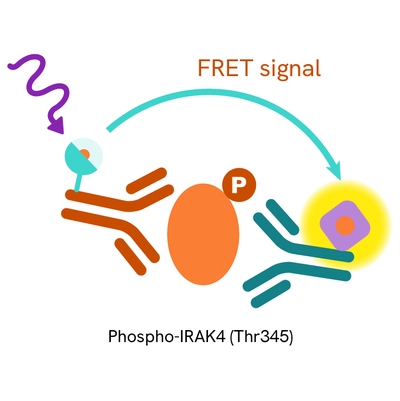
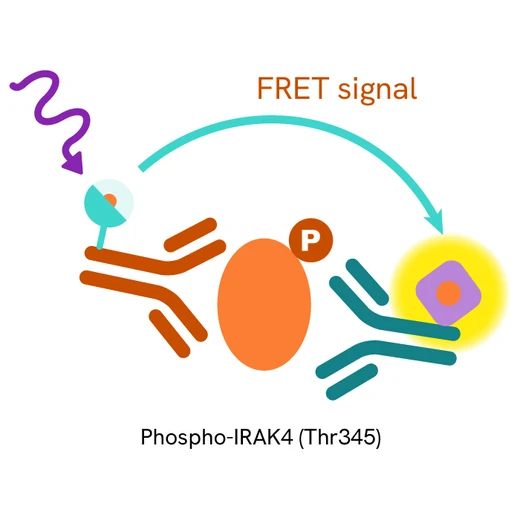
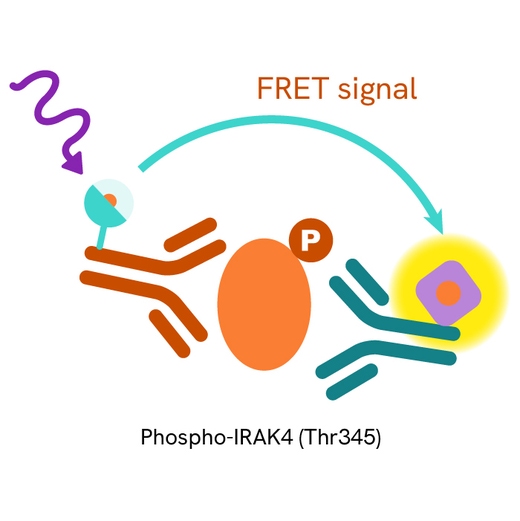
The Phospho-IRAK4 (Thr345) assay measures IRAK4 when phosphorylated at Thr345. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The assay uses 2 antibodies, one labeled with a donor fluorophore and the other with an acceptor. The first antibody was selected for its specific binding to the phosphorylated motif on the protein, and the second for its ability to recognize the protein independently of its phosphorylation state. Protein phosphorylation leads to an immune-complex formation involving both labeled antibodies, which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample and provides a means of assessing the protein's phosphorylation state under a no-wash assay format.

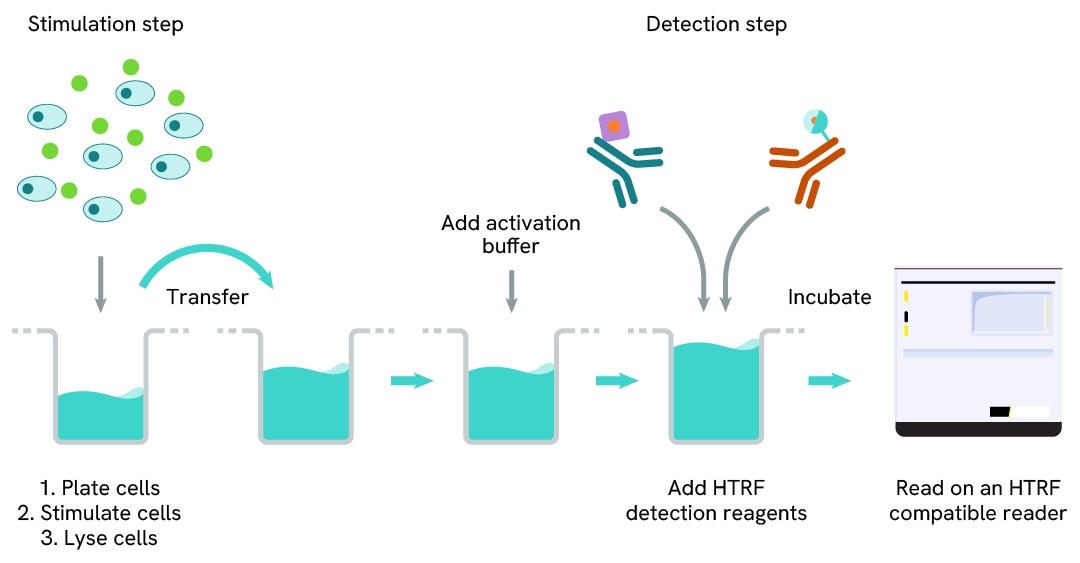
Phospho-IRAK4 (Thr345) two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of Phospho-IRAK4 (Thr345) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.
Assay validation
Induction of phospho-IRAK4 (Thr345) in cellular model
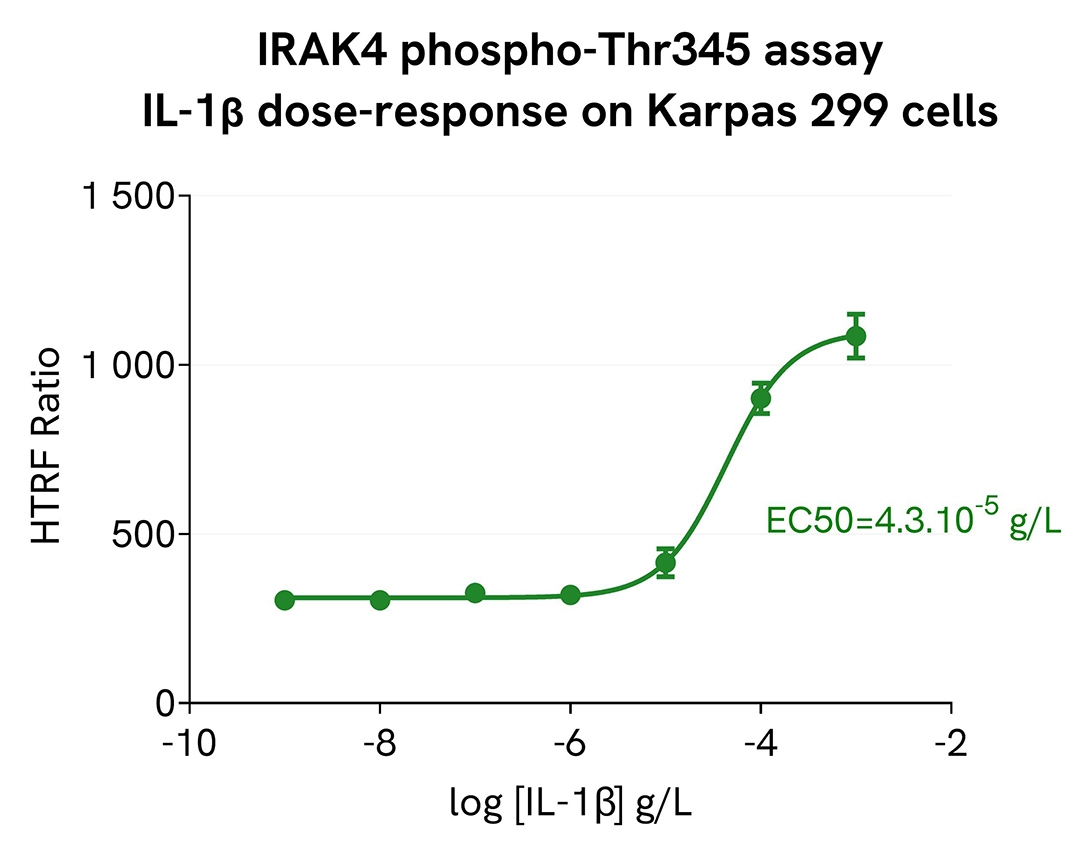
The experiments were performed using the two-plate assay protocol for suspension cells. Karpas-299 cells were plated in 96-well half area culture plates at a density of 400,000 cells/well and treated with increasing concentrations of IL-1b.
After 45 minutes of incubation at 37°C, 5% CO2, 10µL of supplemented Lysis Buffer#1 (4X) were dispensed into each well and left for 30min.
For the detection, 14 µL of lysates were transferred into a ProxiPlate-384 (# 6008280/9), before the addition of 2µL of activation buffer followed by 4 µL of HTRF Phospho-IRAK4 (Thr345) detection reagents. HTRF signals were recorded after an overnight incubation at RT.
As expected, IL-1b triggered a dose-dependent increase in the level of Phospho-IRAK4 (Thr345).
Inhibition of phospho-IRAK4 (Thr345) in cellular model
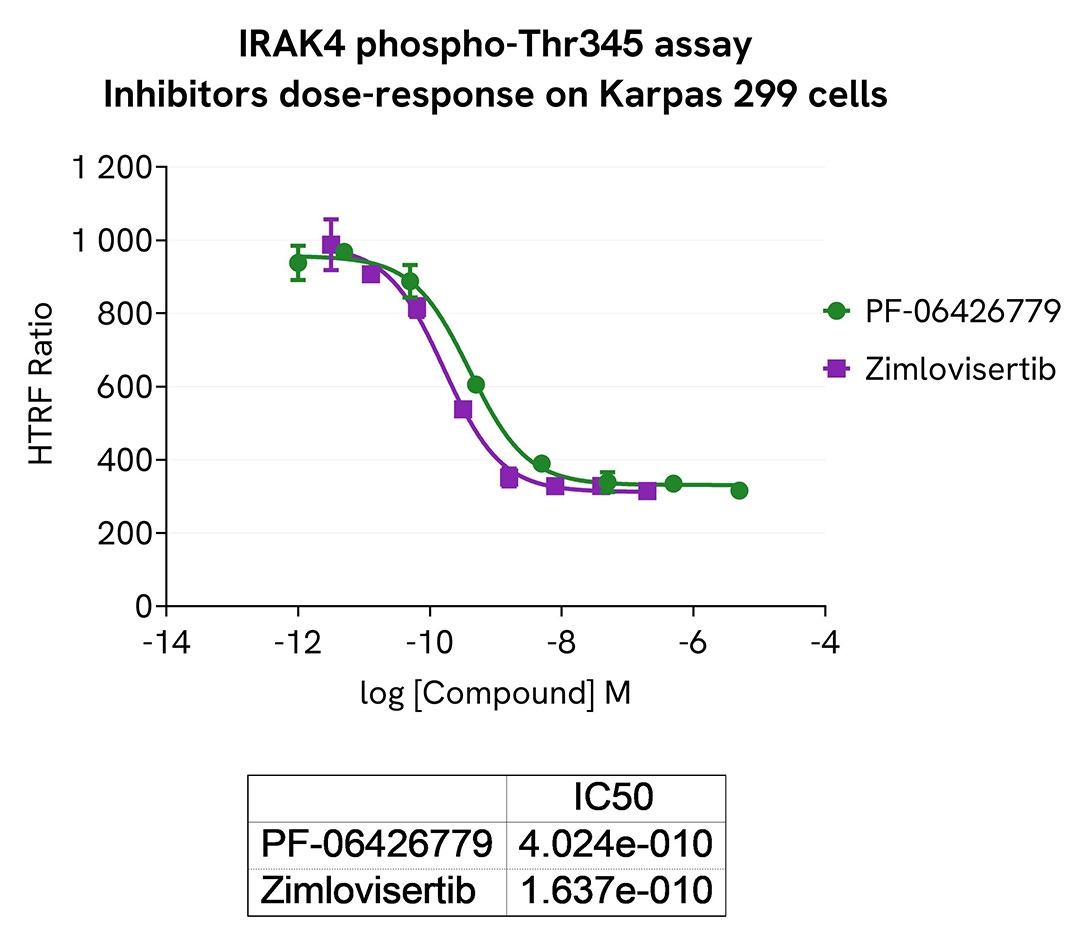
The experiments were performed using the two-plate assay protocol for suspension cells. Karpas-299 cells were plated in 96-well half area culture plates at a density of 400,000 cells/well and stimulated with 100 ng/mL IL-1b for 45 min to increase the level of Phospho-IRAK4 (Thr345). Then increasing concentrations of two IRAK4 inhibitors were added. After 45 minutes of incubation at 37°C, 5% CO2, 10µL of supplemented Lysis Buffer#1 (4X) were dispensed into each well and left for 30min.
For the detection, 14 µL of lysates were transferred into a ProxiPlate-384 (# 6008280/9), before the addition of 2µL of activation buffer followed by 4 µL of HTRF Phospho-IRAK4 (Thr345) detection reagents. HTRF signals were recorded after an overnight incubation at RT.
As expected, both inhibitors triggered a dose-dependent decrease in the level of Phospho-IRAK4 (Thr345).
HTRF IRAK4 Phospho-T345 assay compared to Western Blot
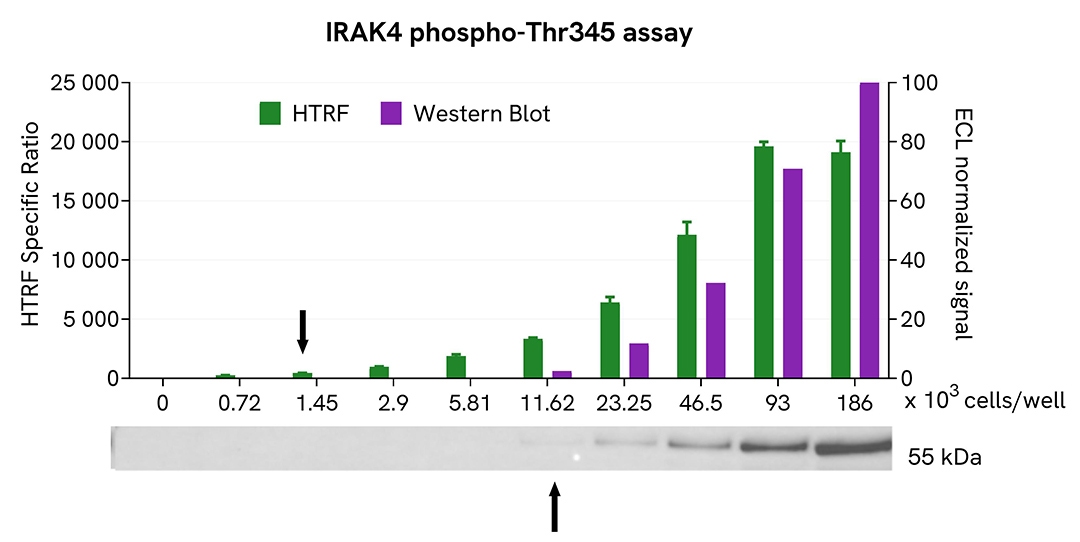
U937 cells were grown in a T175 flask in complete culture medium at 37°C, 5% CO2. After centrifugation and medium removal, cells were lysed with 3 mL of supplemented lysis buffer (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 14 µL of each dilution were transferred into a low volume white microplate before the addition of 2µL of activation buffer, followed by 4 µL of HTRF Phospho-IRAK4 (Thr345) detection reagents. Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
Using the Phospho-IRAK4 (Thr345) assay, 1450 cells/well were enough to detect a significant signal, while 11600 cells were needed to obtain a minimal chemiluminescent signal using Western Blot. Therefore, in these conditions, the Phospho-IRAK4 (Thr345) assay was 8 times more sensitive than the Western Blot technique.
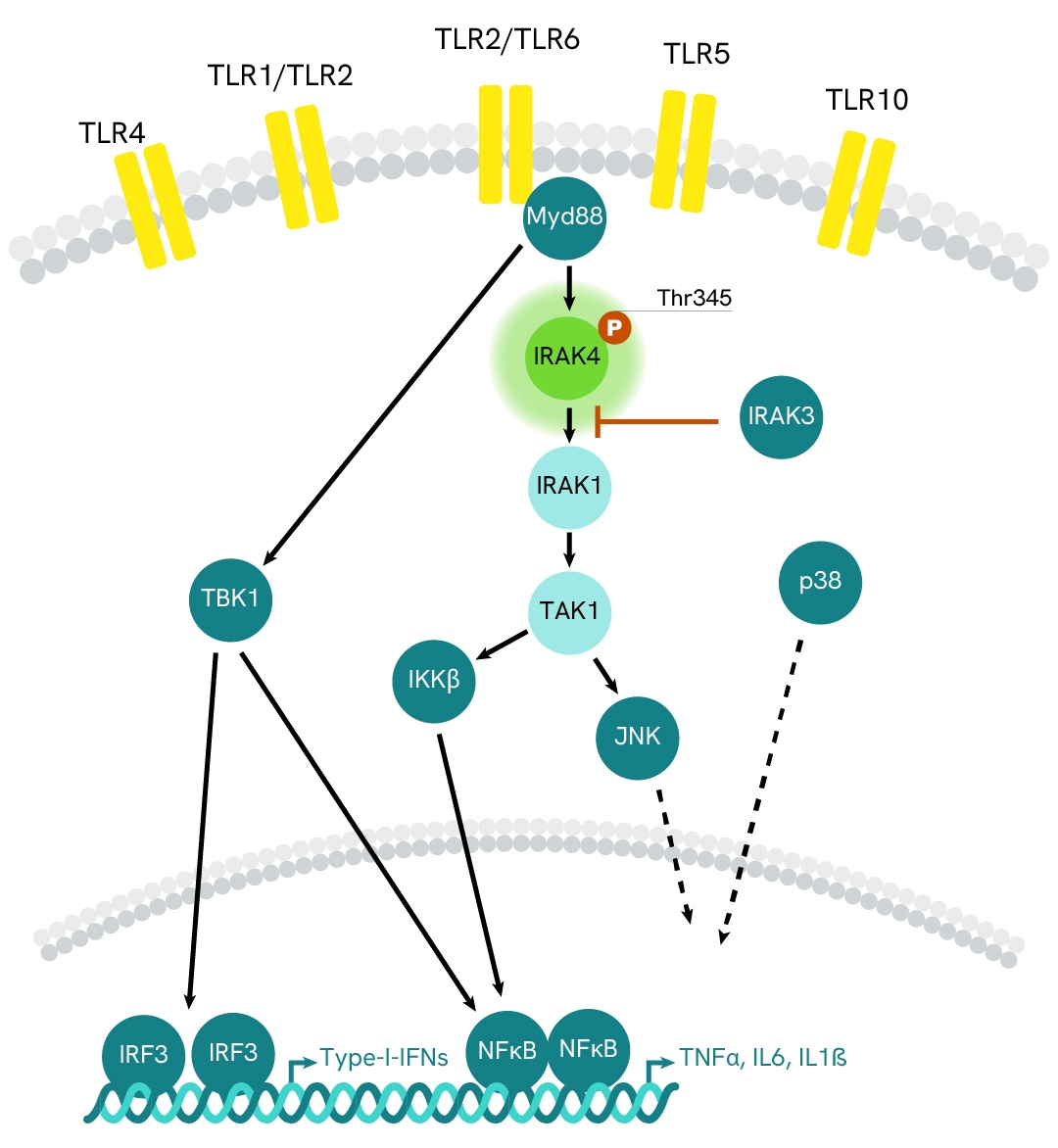
Simplified pathway
IRAK4 signaling pathway
IRAK4 is a protein kinase belonging to the interleukin-1 receptor-associated kinases (IRAKs), which are involved in signaling pathways transduced by the Toll/Interleukin-1 receptor superfamily upon PAMP recognition. Like IRAK2 and IRAK1, IRAK4 signals through TRAF6, leading to the activation of the NFκB and MAPK pathways, and resulting in the transcription of pro-inflammatory cytokines. Unlike other IRAK proteins, the suppression of IRAK4 activity completely impairs TLR receptor signaling, making it a pivotal component in the IL-1/TLR signaling pathway.
SDS, COAs, Manuals and more
Are you looking for technical documents related to the product? We have categorized them in dedicated sections below. Explore now.
- LanguageEnglishCountryUnited States
- LanguageFrenchCountryFrance
- LanguageGermanCountryGermany
- Resource TypeManualLanguage英语Country-


How can we help you?
We are here to answer your questions.







































