

NEXTFLEX Rapid Directional RNA-Seq Kit 2.0

NEXTFLEX Rapid Directional RNA-Seq Kit 2.0


| Feature | Specification |
|---|---|
| Automation Compatible | Yes |
| Product Group | RNA-seq |



Product information
Overview
- High coverage uniformity with very low duplicate reads
- Input flexibility – 5 ng – 5 µg total RNA or 5 ng – 1 µg rRNA-depleted RNA
- Reverse-transcriptase and cleanup/size-selection beads are supplied in-kit
- Tested with NEXTFLEX Poly(A) Beads 2.0 and NEXTFLEX RiboNaut rRNA Depletion for directional detection of coding and non-coding transcripts (including lncRNA)
- Works with NEXTFLEX RNA-Seq 2.0 UDI barcodes for 2 – 384-plex runs or 96-plex UDI-UMI adapters to curb PCR duplicates
- Automation-ready
- Compatible with Illumina® and Element Biosciences™ AVITI™ sequencers
Additional product information

Performance of Poly(A)-selected Libraries
Figure 1. The NEXTFLEX Rapid Directional RNA-Seq kit 2.0 demonstrates even coverage along transcripts compared to the Competitor N kit.
Poly(A) mRNA was isolated from Universal Human Reference RNA (Agilent #740000) using the NEXTFLEX Poly(A) beads 2.0 and the Competitor N Poly(A) enrichment kit. Libraries were generated using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0 and the Competitor N’s library preparation kit. The resulting libraries were sequenced on the Illumina MiSeq® sequencer using paired-end mode (2×76 bp). Reads were trimmed using cutadapt and mapped to the Gencode v30 reference using bowtie2. The coverage along transcripts was calculated using the BBMap pileup tool.
Figure 2. The NEXTFLEX Rapid Directional RNA-Seq kit 2.0 demonstrate low duplication rate compared to the Competitor N kit.
Poly(A) mRNA was isolated from Universal Human Reference RNA (Agilent ®#740000) using the NEXTFLEX Poly(A) beads 2.0 and the Competitor N Poly(A) enrichment kit. Libraries were generated using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0 and the Competitor N’s library preparation kit. The resulting libraries were sequenced on the Illumina MiSeq sequencer using paired-end mode (2×76 bp). Reads were trimmed using cutadapt, mapped to the Gencode v30 reference using bowtie2, and randomly downsampled to 100k reads. Duplication rate was calculated using the fastp all-in-one FASTQ preprocessor.
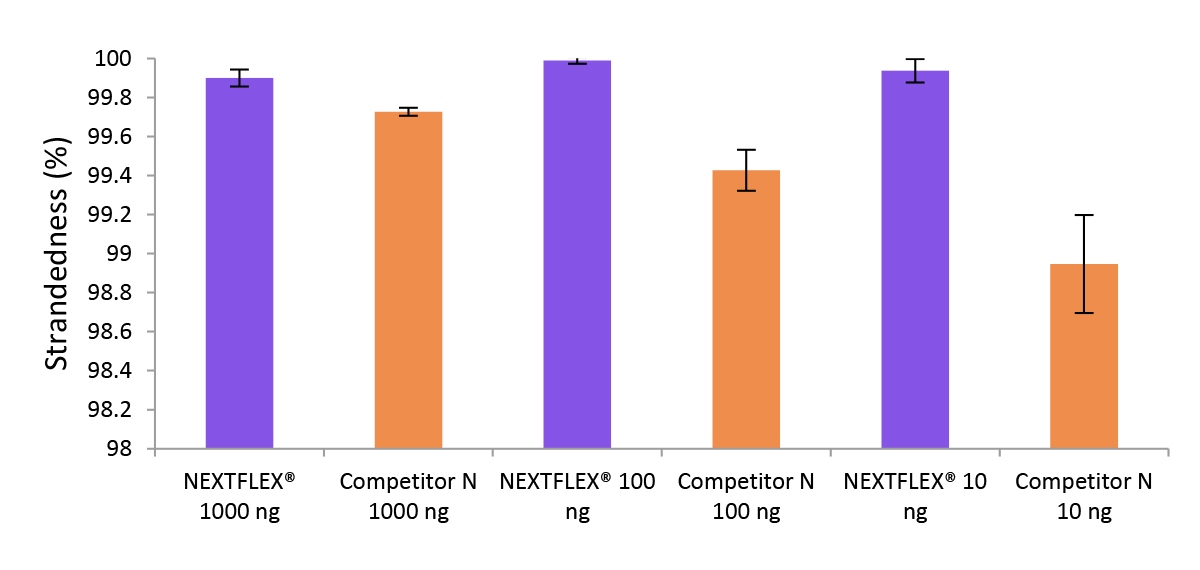
Figure 3. The NEXTFLEX Rapid Directional RNA-Seq kit 2.0 demonstrates comparable directionality to the Competitor N kit.
Poly(A) mRNA was isolated from Universal Human Reference RNA (Agilent #740000) containing ERCC RNA Spike-In mix (Thermo Fisher Scientific #4456740) using the NEXTFLEX Poly(A) Beads 2.0 and the Competitor N Poly(A) enrichment kit. Libraries were generated using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0 and the Competitor N’s library preparation kit. The resulting libraries were sequenced on the Illumina MiSeq sequencer using paired-end mode (2×76 bp). Reads were trimmed using cutadapt and mapped to the ERCC92 reference using bowtie2. Strandedness was calculated using SAMtools.
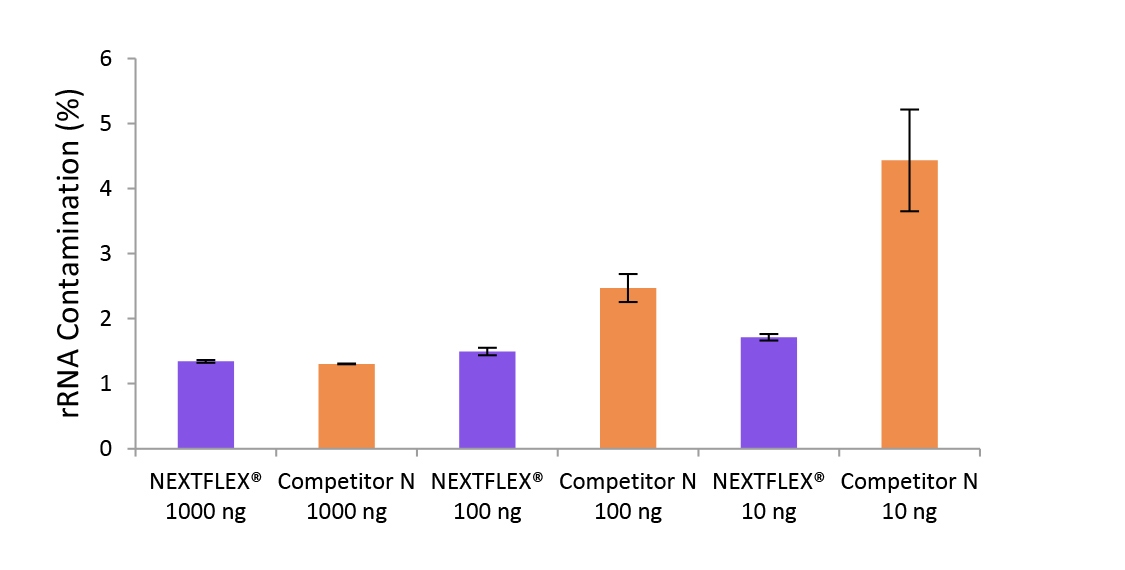
Figure 4. The NEXTFLEX Rapid Directional RNA-Seq kit 2.0 delivers libraries containing low levels of rRNA contamination than the Competitor N kit.
Poly(A) mRNA was isolated from Universal Human Reference RNA (Agilent #740000) using the NEXTFLEX Poly(A) Beads 2.0 and the Competitor N Poly(A) enrichment kit. Libraries were generated using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0 and the Competitor N’s library preparation kit. The resulting libraries were sequenced on the Illumina MiSeq sequencer using paired-end mode (2×76 bp). The reads were trimmed using cutadapt and the percent of rRNA was determined by using bowtie2 to map reads to human rRNA. The NEXTFLEX Rapid Directional RNA-Seq kit 2.0 demonstrated superior removal of 5S, 5.8S, 12S, 16S, 18S, and 28S rRNA species compared to the Competitor N kit.
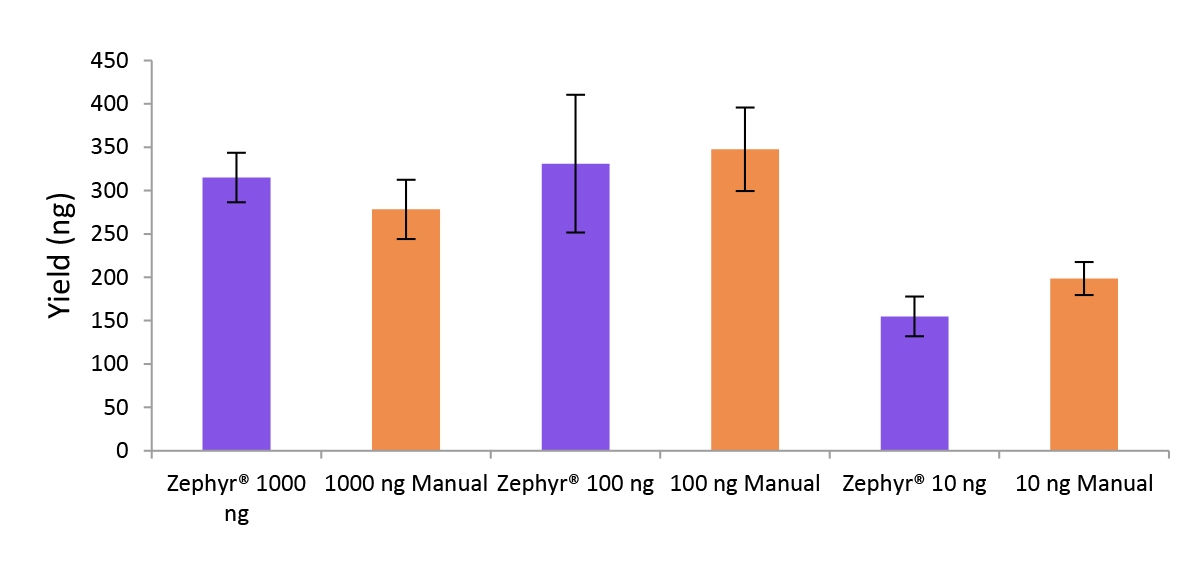
Figure 5. Libraries prepared using the Zephyr G3 NGS workstation and manually deliver comparable yields using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0.
Poly(A) mRNA was isolated from Universal Human Reference RNA (Agilent #740000) using the NEXTFLEX Poly(A) Beads 2.0. Libraries were generated using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0. Final library concentrations were quantified using the Qubit® 2.0 fluorometer (Thermo Fisher® Scientific #Q32866).
Performance of rRNA-depleted Libraries
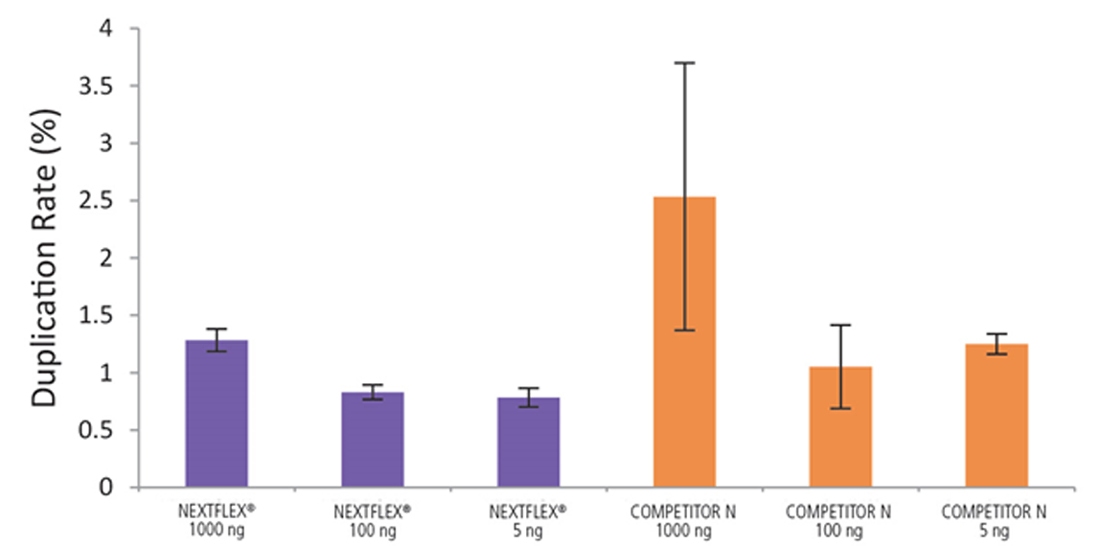
Figure 6. The NEXTFLEX Rapid Directional RNA-Seq kit 2.0 demonstrate low duplication rates compared to the Competitor N kit. rRNA-depleted total RNA was isolated from Universal Human Reference RNA (Agilent #740000) using the NEXTFLEX RiboNaut rRNA depletion kit (human, mouse, rat) and the Competitor N rRNA-depletion kit. Libraries were generated using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0 and the Competitor N’s library preparation kit. The resulting libraries were sequenced on the Illumina MiSeq sequencer using single-end mode (1×151 bp). Reads were trimmed using cutadapt, mapped to the Gencode v30 reference transcriptome using bowtie2, and randomly downsampled to 28k reads. Duplication rate was calculated using the fastp all-in-one FASTQ preprocessor.
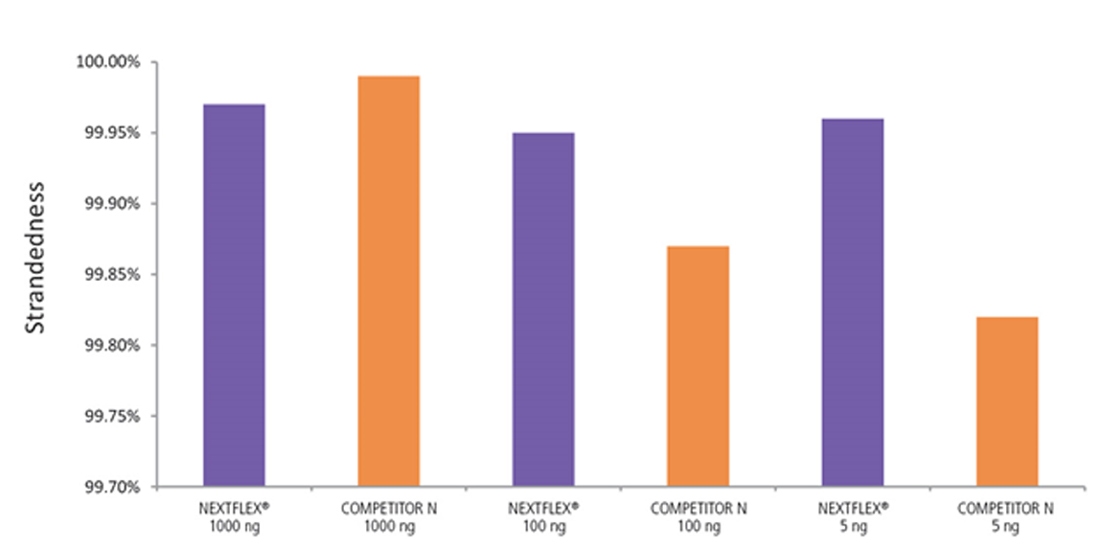
Figure 7. The NEXTFLEX Rapid Directional RNA-Seq kit 2.0 demonstrates comparable directionality relative to the Competitor N kit. rRNA-depleted total RNA was isolated from Universal Human Reference RNA (Agilent #740000) using the NEXTFLEX RiboNaut rRNA depletion kit (human, mouse, rat) and the Competitor N rRNA-depletion kit. Libraries were generated using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0 and the Competitor N’s library preparation kit. The resulting libraries were sequenced on the Illumina MiSeq sequencer using single-end mode (1×151 bp). Reads were trimmed using cutadapt and mapped to the Gencode v30 reference transcriptome using bowtie2. Reads from respective samples were combined and downsampled for a total of 800k reads each. Strandedness was calculated using the fastp all-in-one FASTQ preprocessor.
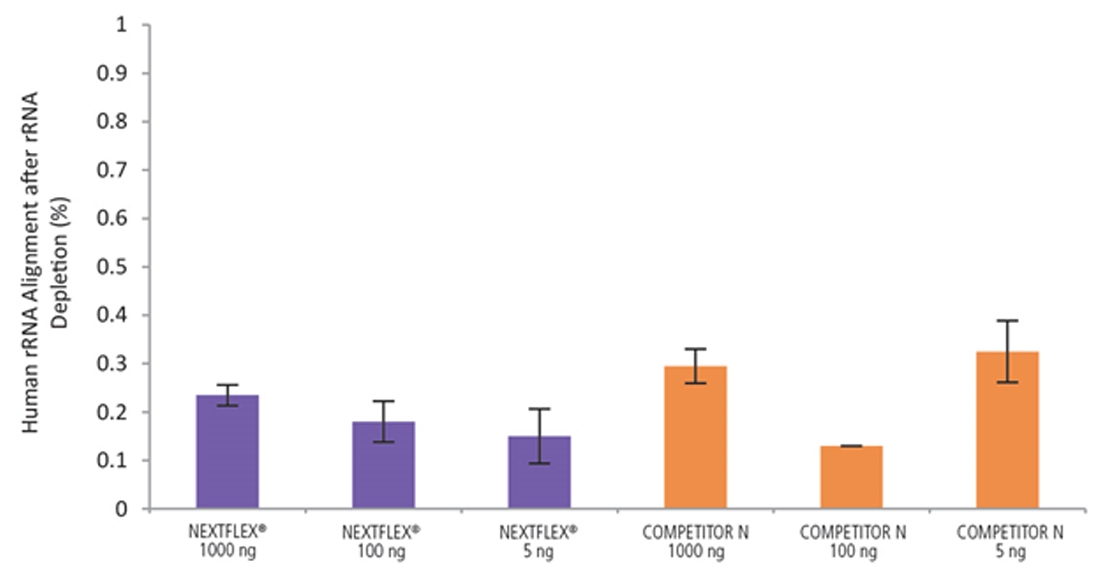
Figure 8. The NEXTFLEX Rapid Directional RNA-Seq kit 2.0 delivers libraries containing low levels of rRNA contamination compared to the Competitor N kit. rRNA-depleted total RNA was isolated from Universal Human Reference RNA (Agilent #740000) using the NEXTFLEX RiboNaut rRNA depletion kit (human, mouse, rat) and the Competitor N rRNA-depletion kit. Libraries were generated using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0 and the Competitor N’s library preparation kit. The resulting libraries were sequenced on the Illumina MiSeq sequencer using single-end mode (1×151 bp). The reads were trimmed using cutadapt and the percent of rRNA was determined by using bowtie2 to map reads to human rRNA. The NEXTFLEX Rapid Directional RNA-Seq kit 2.0 demonstrated superior removal of 5S, 5.8S, 12S, 16S, 18S, and 28S rRNA species compared to the Competitor N kit.
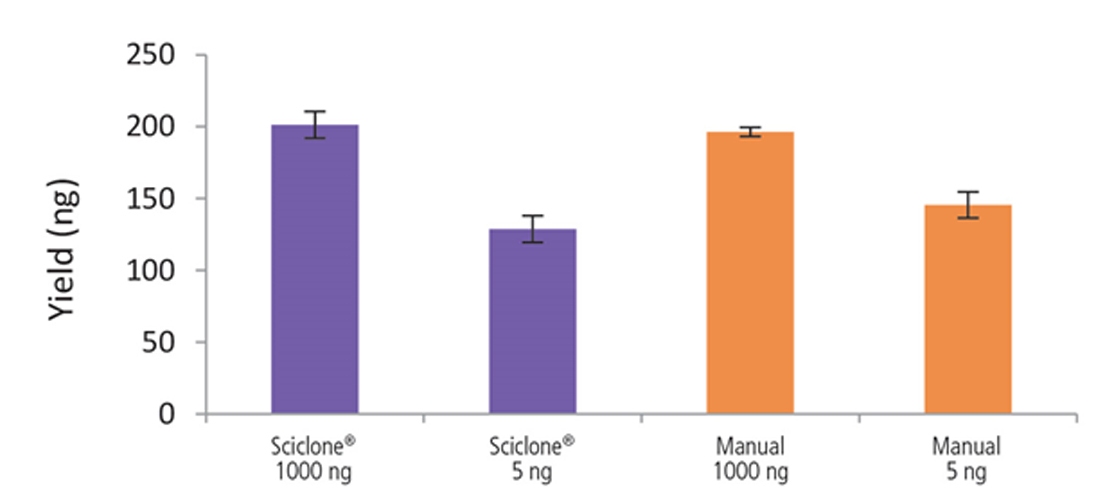
Figure 9. Libraries prepared using the Sciclone G3 NGSx workstation and manually deliver comparable yields using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0. rRNA-depleted total RNA was isolated from Universal Human Reference RNA (Agilent #740000) using the NEXTFLEX RiboNaut rRNA depletion kit. Libraries were generated using the NEXTFLEX Rapid Directional RNA-Seq kit 2.0. Final library concentrations were quantified using the Qubit 2.0 fluorometer (Thermo Fisher Scientific #Q32866).
Explore our Gene Modulation Reagents
Explore our gene modulation reagents to either increase or silence the expression levels of your gene of interest as part of your functional studies.
Specifications
| Automation Compatible |
Yes
|
|---|---|
| Format |
Automation Friendly Volumes
|
| Product Group |
RNA-seq
|
| Shipping Conditions |
Dual Temperature
|
| Unit Size |
96 rxns
|
Citations
- Bond, D.M., Ortega-Recalde, O., Laird, M.K. et al. The admixed brushtail possum genome reveals invasion history in New Zealand and novel imprinted genes. Nat Commun 14, 6364 (2023). doi.org/10.1038/s41467-023-41784-8.
- Gaonkar, C.C., Campbell, L. De novo transcriptome assembly and gene annotation for the toxic dinoflagellate Dinophysis. Sci Data 10, 345 (2023). doi.org/10.1038/s41597-023-02250-8.
- Laudadio, I., Carissimi, C., Scafa, N. et al. Characterization of patient-derived intestinal organoids for modelling fibrosis in Inflammatory Bowel Disease. Inflamm. Res. 73, 1359–1370 (2024). https://doi.org/10.1007/s00011-024-01901-9.
- Manukjan, N., Chau, S., Caiment, F. et al. Wnt7a Decreases Brain Endothelial Barrier Function Via β-Catenin Activation. Mol Neurobiol 61, 4854–4867 (2024). doi.org/10.1007/s12035-023-03872-0
- Mulroney, T.E., Pöyry, T., Yam-Puc, J.C. et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 625, 189–194 (2024). doi.org/10.1038/s41586-023-06800-3
- Smith, C. H., Mejia-Trujillo, R., Breton, S., Pinto, B. J., Kirkpatrick, M., & Havird, J. C. (2023). Mitonuclear Sex Determination? Empirical Evidence from Bivalves. Molecular Biology and Evolution, 40(11), msad240.
- Tóvári, J.; Vári-Mező, D.; Surguta, S.E.; Ladányi, A.; Kigyós, A.; Cserepes, M. Evolving Acquired Vemurafenib Resistance in a BRAF V600E Mutant Melanoma PDTX Model to Reveal New Potential Targets. Cells 2023, 12, 1919. doi.org/10.3390/cells12141919.
- Higdon, A. L., Chemmama, I. E., Yao, T., … & Inada, T. (2024). Truncated protein isoforms generate diversity of protein localization and function in yeast. Cell Systems, 15(4), 388–408.e4. https://doi.org/10.1016/j.cels.2024.03.005
- García-Santamarina, S., Lemos, T., & Thion, M. (2023). Differential expression analysis reveals a new quaternary ammonium compound resistance gene in Serratia sp. HRI. Microorganisms, 11(8), 2045. https://doi.org/10.3390/microorganisms11082045
- Chen, C., Tjeng, R., & Mueller, J. (2024). De novo transcriptome assembly and gene annotation for the toxic dinoflagellate Karenia. Scientific Data, 11, 34. https://doi.org/10.1038/s41597-024-00834-1
- Kirio K, Patop I L, Martin Anduaga A, Harris J M, Pamudurti N, Su T N, Martel C, Kadener S. (2025) Circular RNAs exhibit exceptional stability in the aging brain and serve as reliable age and experience indicators. Cell Reports 44 (4): 115485. https://doi.org/10.1016/j.celrep.2025.115485.
FAQs
-
What types of RNAs are going to be detected with the NEXTFLEX Rapid Directional RNA-seq Kit 2.0 library?
-
What is the starting material I need to use to prepare libraries with this kit?
-
Are the libraries produced by NEXTFLEX Rapid Directional RNA-seq 2.0 stranded?
-
Is this kit compatible with RNA enrichment?
-
Do you offer solutions for depletion of rRNA, globin and others?
-
Is this kit compatible with FFPE-derived/low-quality RNA?
-
Can this kit be used to study long non-coding RNA?
-
Can this kit be used to study circular RNA?
-
What kind of barcodes can be used with the NEXTFLEX Rapid Directional RNA-seq 2.0?
-
Does this kit support long-read sequencing?
-
What are the recommended settings for sequencing?
-
What is the number of reads required for RNA sequencing?
-
What do I need to do to run the NEXTFLEX Rapid Directional RNA 2.0 libraries on the AVITI™ sequencer from Element® Biosciences?
Resources
Are you looking for resources, click on the resource type to explore further.
This flyer describes the benefits of the NEXTFLEX® Rapid Directional RNA-seq 2.0 Kit.
This flyer illustrates the breadth of the NEXTFLEX RNA-seq Portfolio


How can we help you?
We are here to answer your questions.






























